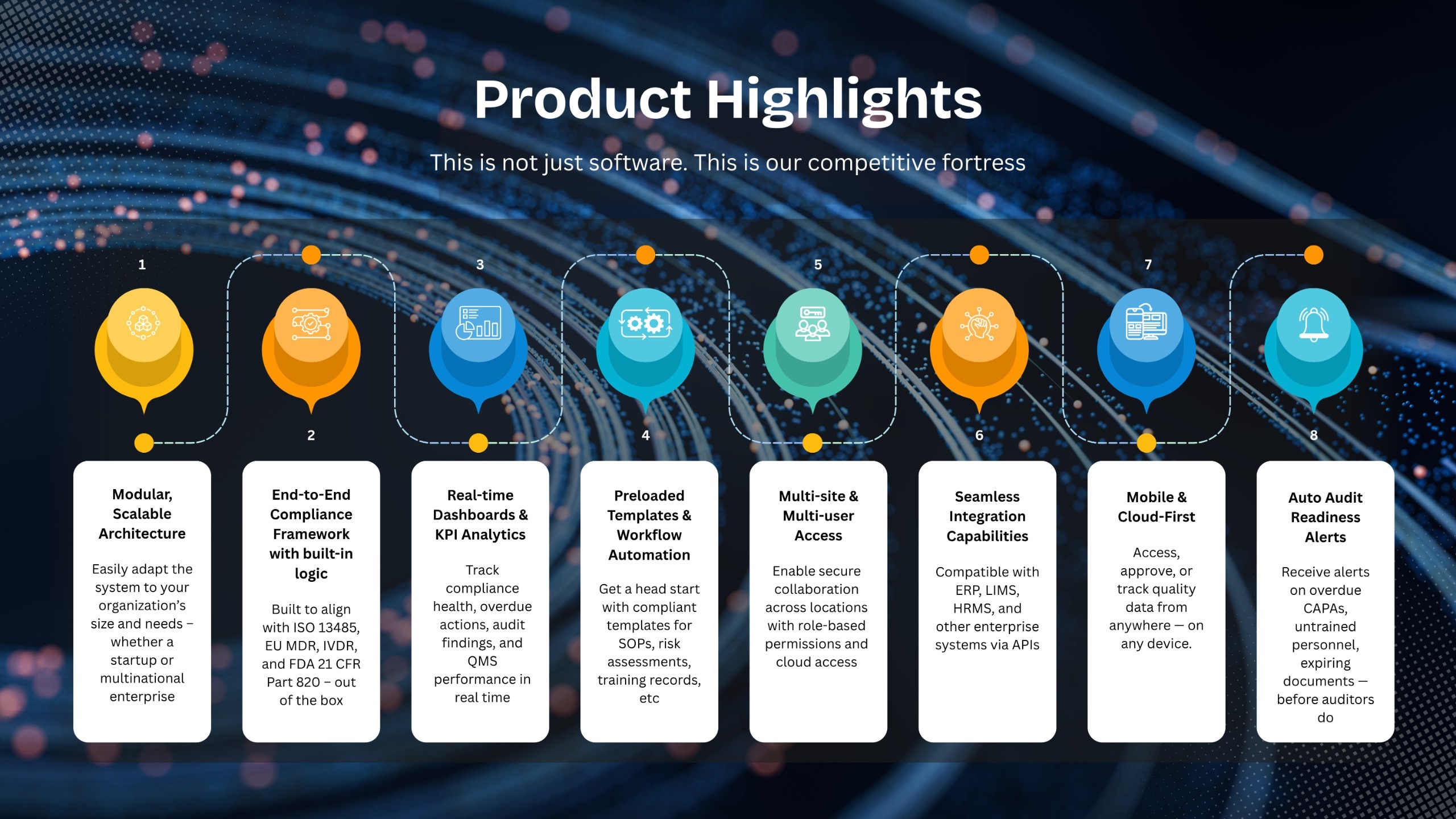
Designed exclusively for Medical devices by Regulatory & QMS experts
eQMS Solution with end-to-end compliance to ISO 13485
Compliance Shouldn’t Be a Burden. It Should Be Built-In.






Unlock Potential
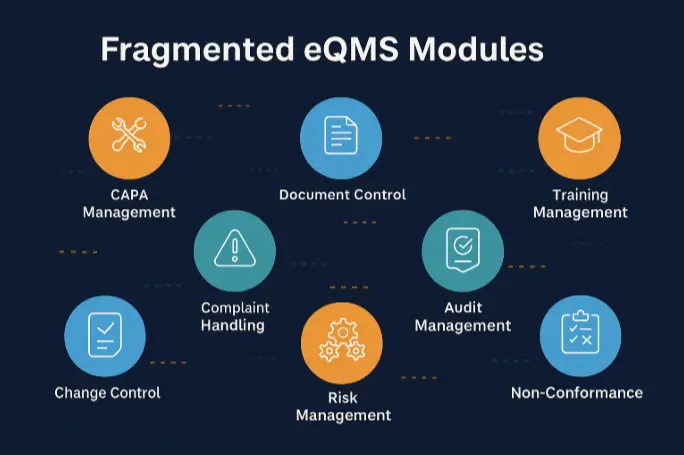
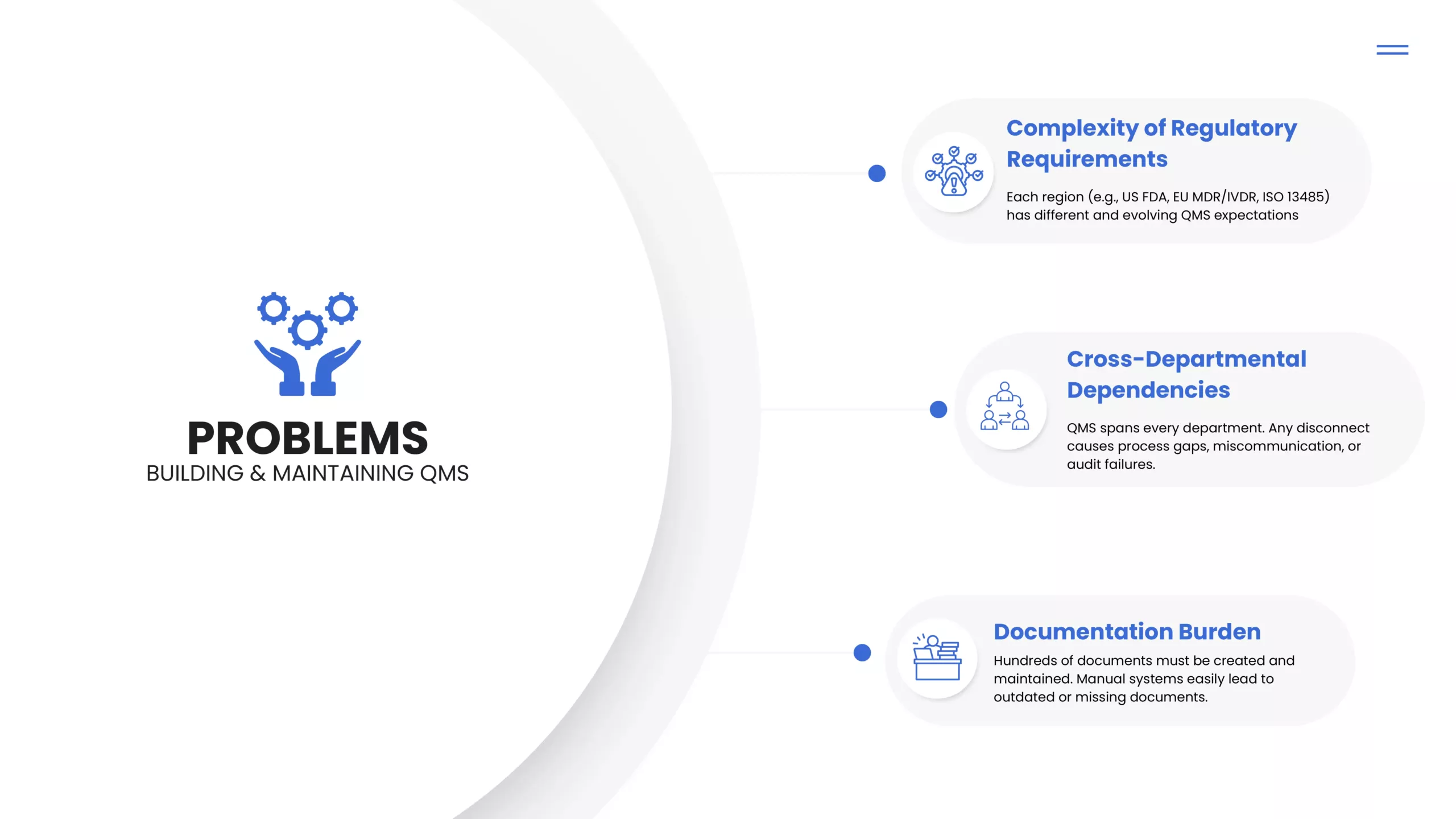
Gaps in current practices & eQMS systems that Regtech aims to fill
Unlike current eQMS tools that operate with disconnected modules, siloed data, and no real-time cross-departmental sync often requiring manual cross-linking and leaving teams unprepared for inspections, Regtech delivers a unified, audit-ready platform built for seamless collaboration and compliance that mirrors the cross-functional reality of ISO 13485. Rather than isolating workflows into rigid modules, Regtech offers seamless, logic-driven processes that unify all departmental processes within a single intelligent system.Trusted by startups and scaleups to simplify compliance in the most customisable format.

Disconnected individual modules with real-time cross-departmental sync
Existing eQMS solutions lack the traceability and inter-departmental communication that a software solution should provide.
Manual cross-linking of data that is siloed
Such fragmented solutions lead to manual dependency and again putting the systems into the same loop where the organisation faces weak inspection readiness
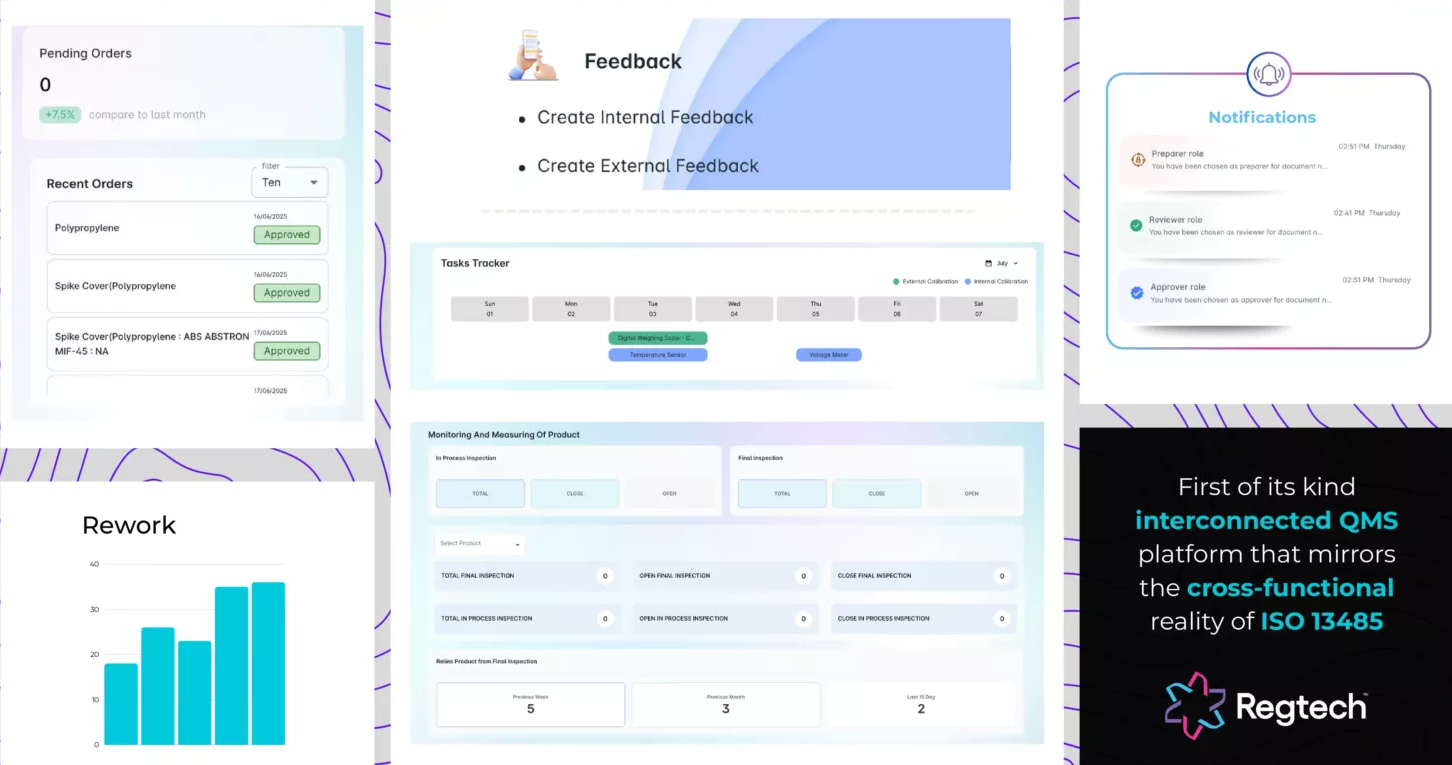
Unified System, Not Fragmented Modules
“Compliance doesn’t work in silos, and neither does Regtech.”
Regtech is designed as a first-of-its-kind, holistic, interconnected QMS platform that mirrors the cross-functional reality of ISO 13485. Rather than isolating workflows into rigid modules, Regtech offers seamless, logic-driven processes that unify all departmental processes within a single intelligent system.

Potential customers found
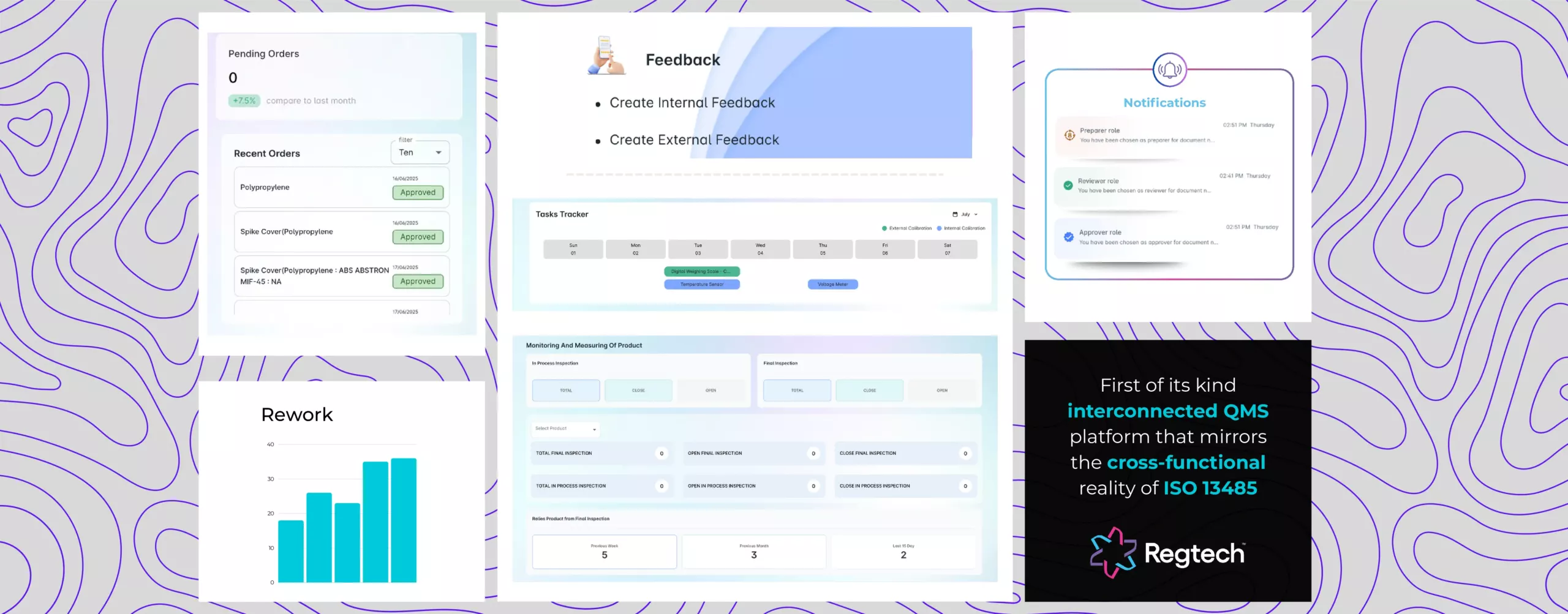
Unified System, Not Fragmented Modules
“Compliance doesn’t work in silos, and neither does Regtech.”
Regtech is designed as a first-of-its-kind, holistic, interconnected QMS platform that mirrors the cross-functional reality of ISO 13485. Rather than isolating workflows into rigid modules, Regtech offers seamless, logic-driven processes that unify all departmental processes within a single intelligent system.


Potential customers found



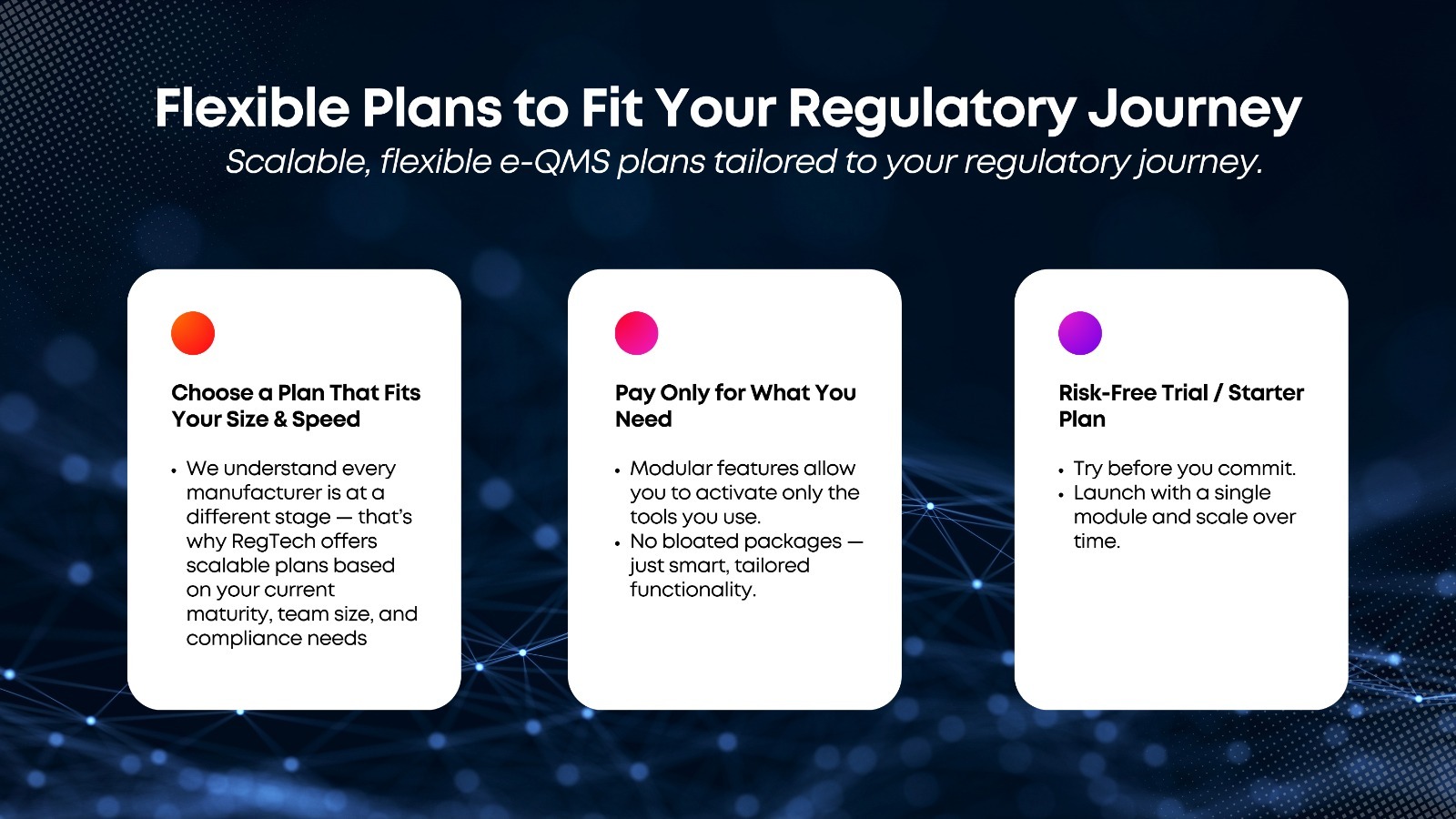
See RegTech in Action
Make your QMS Smart: Let us show you how RegTech simplifies compliance, without compromising control.
Sed ut perspiciatis unde omnis iste natus ut perspic iatis unde omnis iste perspiciatis ut perspiciatis unde omnis iste natus. Sed ut perspiciatis unde omnis iste natus ut perspic iatis unde omnis iste perspiciatis.
Comes preloaded with validated templates (SOPs, Manual, Registers) to drastically reduce onboarding time and ensure instant compliance alignment -
0+

Made by 100+ Compliance experts
Solution tailored for MedTech industries
0%
Audit Ready
At Maven, we’ve helped manufacturers across 20+ countries navigate CE, FDA, UKCA, ISO, MDSAP and country-specific regulatory hurdles.
But no matter how good the strategy was, the execution bottleneck always came from one place: The QMS.
That’s when we realized that true compliance needs more than SOPs. It needs a system that understands regulatory logic, adapts to changing norms, and empowers teams without overloading them.
Yogesh Dad
Director, Regtech

Digital insights
Latest news and insights from the industry
The Hidden Cost of Non-Integrated QMS Tools: What You’re Not Calculating
Common Questions
Frequently asked questions
What is an eQMS and why do medical device companies need one?
An eQMS (Electronic Quality Management System) helps medical device companies manage compliance with industry regulations like ISO 13485 and FDA 21 CFR Part 11. It centralizes quality processes like CAPA, document control, audits, training, and risk management—making your systems audit-ready, traceable, and efficient. Without an eQMS, you're stuck juggling spreadsheets, emails, and siloed tools that slow you down and put compliance at risk.
How is RegTech different from generic QMS software?
Most QMS tools are one size fits all. RegTech is purpose built for the medical device industry. It's aligned with ISO 13485, FDA 21 CFR Part 820, Part 11, EU MDR, and IVDR — so you're not just customizing workflows, you're starting from compliance. From automated audit trails to ready to use templates, RegTech is a system that thinks like an auditor and works like a quality consultant.
Can startups use RegTech, or is it only for enterprise companies?
RegTech is built to scale. Whether you're a 5 person startup preparing for your first ISO 13485 certification, or a global manufacturer managing multiple sites, RegTech adapts to your size and workflows. You can start lean and grow without switching systems later.
Does RegTech support multisite and multiuser access?
Absolutely. RegTech is built for teams of all sizes, across multiple locations. You get granular user roles, site level permissions, and centralized dashboards that give real time visibility across your entire organization.
How long does it take to implement RegTech?
Most teams are up and running in 2 to 4 weeks. RegTech comes with preloaded templates, guided onboarding, and expert support — so you don’t waste months configuring or migrating. Our goal: get you audit ready without delays.
Is RegTech a cloud based solution?
Yes. RegTech is fully cloud based, mobile ready, and secure. You can access your QMS from anywhere, anytime — whether you're on the shop floor, in a lab, or working remotely.
Can RegTech integrate with our existing tools?
Yes. RegTech offers integration capabilities via secure APIs. It works with ERP systems, HR tools, training platforms, and analytics dashboards. If you need something custom, our team can support that too.
What kind of support do you offer?
We offer onboarding assistance, regulatory consulting, detailed user manual and flow-specific videos. You’ll never be left figuring things out alone.
Can I try RegTech before committing?
Of course. You can book a personalized demo or request a free trial access. See the system in action, test workflows, and get hands on, no commitment required.
How secure is my data with RegTech?
RegTech is hosted on encrypted enterprise grade infrastructure. We follow best practices in data security, including role based access control, secure backups, and two factor authentication. Your data stays protected and compliant.